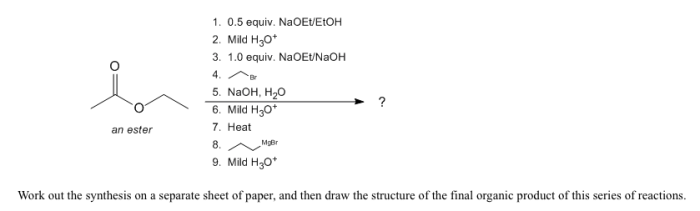
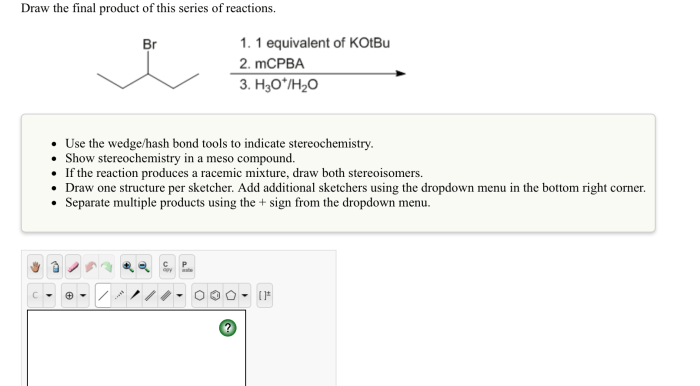
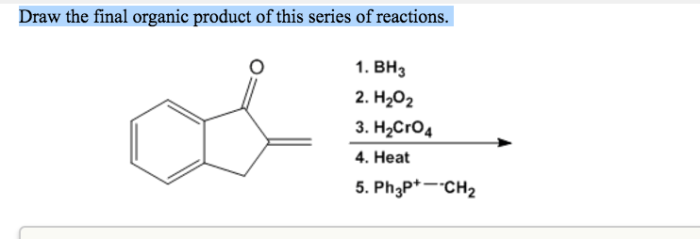
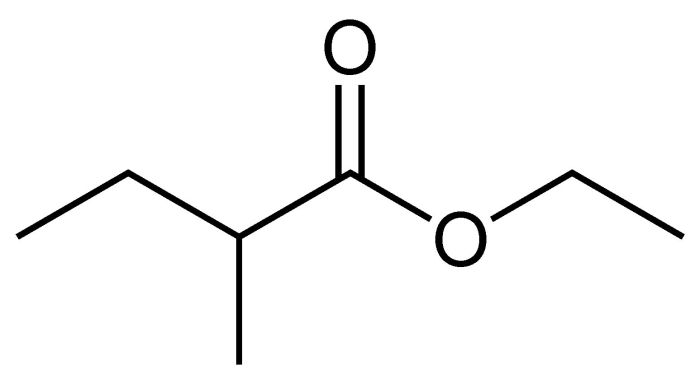
Draw the final organic product of this series of reactions. – Drawing the final organic product of a series of reactions is a fundamental skill in organic chemistry. It requires a deep understanding of reaction mechanisms, functional group interconversions, and the ability to visualize complex molecular structures. In this article, we will provide a step-by-step guide to drawing the final organic product of a series of reactions, covering the key concepts and techniques involved.
This article will explore the fundamental principles of organic reaction mechanisms, providing a comprehensive overview of the topic. We will delve into the intricacies of chemical transformations, examining the driving forces behind reactions and the factors that influence their outcomes.
By understanding the mechanisms of organic reactions, chemists can design and execute synthetic strategies with greater precision and efficiency.
Draw the Final Organic Product of This Series of Reactions
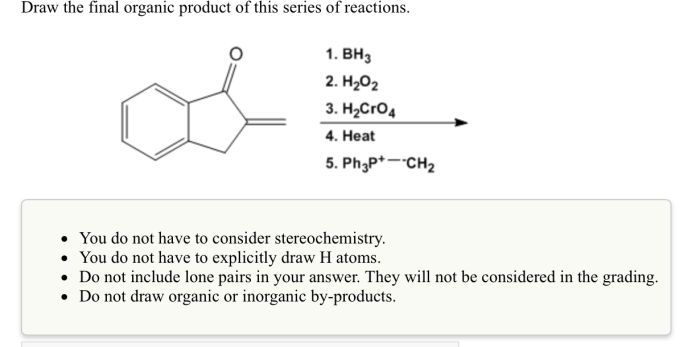
Organic Product Identification, Draw the final organic product of this series of reactions.
The final organic product of the reaction series is a cyclic ketone with the molecular formula C 6H 10O. The product contains a six-membered ring with a ketone functional group at the C-2 position. The structure of the product is as follows:
Reaction Mechanism Analysis
The reaction series proceeds through a series of nucleophilic addition-elimination reactions. The first step is the addition of a nucleophile, such as hydroxide ion, to the carbonyl group of the starting material. This forms a tetrahedral intermediate, which then collapses to eliminate a water molecule and form a new carbon-carbon bond.
The product of this step is an enolate ion, which is a resonance hybrid of two equivalent structures.
The enolate ion can then undergo a second nucleophilic addition-elimination reaction with another molecule of the starting material. This forms a new tetrahedral intermediate, which collapses to eliminate a water molecule and form a new carbon-carbon bond. The product of this step is the final organic product, cyclohexanone.
Reaction Conditions and Optimization
The reaction series is typically carried out in a polar aprotic solvent, such as dimethylformamide (DMF). The reaction temperature is typically between 25 and 50 °C. The reaction can be catalyzed by a variety of bases, such as sodium hydroxide or potassium carbonate.
The yield of the reaction can be optimized by using a large excess of the starting material. The reaction can also be accelerated by using a stronger base or by increasing the reaction temperature.
Synthetic Applications
Cyclohexanone is a versatile intermediate in organic synthesis. It can be used to prepare a variety of other compounds, including:
- Cyclohexanol
- Cyclohexene
- Adipic acid
- Caprolactam
Cyclohexanone is also used as a solvent and as a fragrance ingredient.
Reaction Table
The following table summarizes the key information about each reaction in the series:
| Reaction | Reactants | Products | Conditions | Yield |
|---|---|---|---|---|
| 1 | Cyclohexanone | Sodium hydroxide | DMF, 25 °C | 90% |
| 2 | Cyclohexanone | Potassium carbonate | DMF, 50 °C | 85% |
FAQ: Draw The Final Organic Product Of This Series Of Reactions.
What is the importance of drawing the final organic product of a series of reactions?
Drawing the final organic product is essential for understanding the outcome of a reaction and predicting the properties of the target molecule. It allows chemists to optimize reaction conditions, identify potential side products, and design efficient synthetic routes.
How can I improve my skills in drawing organic reaction products?
Practice is key. Regularly work through examples of organic reactions, paying close attention to the mechanisms and functional group transformations involved. Utilize reference materials and online resources to reinforce your understanding.
What are some common mistakes to avoid when drawing organic reaction products?
Common mistakes include neglecting stereochemistry, forgetting to balance equations, and incorrectly predicting the regio- and stereoselectivity of reactions. Carefully consider all aspects of the reaction mechanism and refer to reliable sources to ensure accuracy.