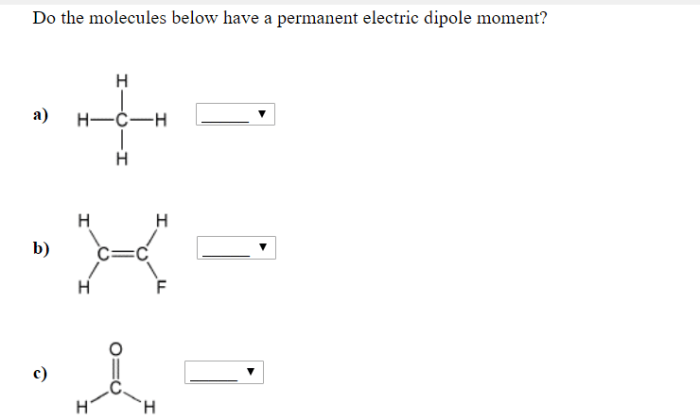
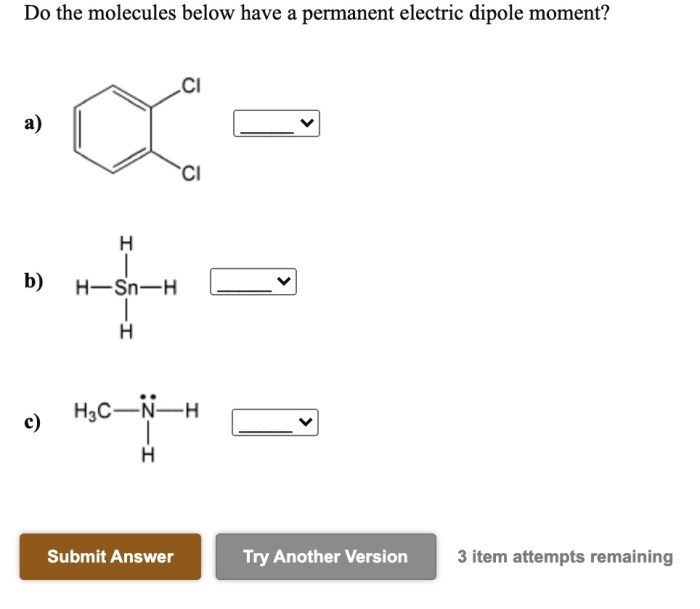
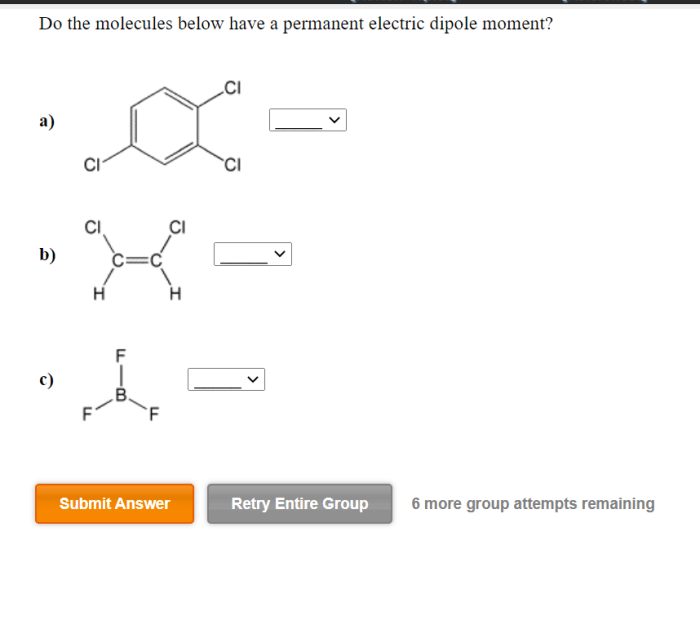
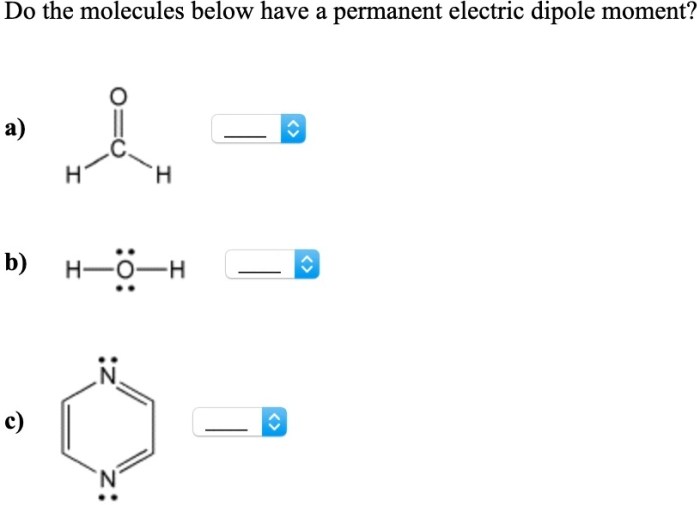
Do the molecules below have a permanent electric dipole moment? This question delves into the realm of molecular polarity, a fundamental concept in chemistry that governs the behavior of molecules in various physical and chemical processes. By examining molecular geometry, electronegativity, resonance, and intermolecular forces, we embark on a journey to unravel the factors that determine the polarity of molecules and their subsequent impact on their properties and interactions.
The concept of molecular polarity stems from the uneven distribution of electrons within a molecule, resulting in the formation of a dipole moment. A permanent electric dipole moment arises when the center of positive charge and the center of negative charge do not coincide, creating a separation of charges within the molecule.
This separation can be attributed to several factors, including the molecular geometry, the electronegativity of the constituent atoms, and the presence of resonance structures.
Molecular Geometry: Do The Molecules Below Have A Permanent Electric Dipole Moment
Molecular geometry refers to the three-dimensional arrangement of atoms within a molecule. It significantly influences the polarity of the molecule by determining the distribution of electrons.
Molecules with symmetrical shapes, such as linear or tetrahedral, tend to have a uniform distribution of electrons and are generally nonpolar. In contrast, molecules with asymmetrical shapes, such as bent or trigonal pyramidal, can exhibit polarity due to uneven electron distribution.
Electronegativity and Bond Polarity
Electronegativity is the ability of an atom to attract electrons towards itself. When atoms with different electronegativities form a bond, the more electronegative atom draws the shared electrons closer, creating a polar covalent bond.
- Polar bonds:Bonds formed between atoms with significant electronegativity differences, resulting in a partial positive charge on one atom and a partial negative charge on the other.
- Nonpolar bonds:Bonds formed between atoms with similar electronegativities, resulting in an equal sharing of electrons and no significant charge separation.
Dipole Moments, Do the molecules below have a permanent electric dipole moment
Electric dipole moment is a measure of the polarity of a molecule. It is calculated as the product of the magnitude of the partial charges and the distance between them.
| Molecule | Dipole Moment (D) |
|---|---|
| H2O | 1.85 |
| NH3 | 1.47 |
| CO2 | 0 |
Molecules with a nonzero dipole moment are considered polar, while those with a zero dipole moment are nonpolar.
Resonance and Delocalization
Resonance occurs when a molecule can be represented by multiple Lewis structures. In such cases, the electrons are delocalized over several atoms, reducing the polarity of the molecule.
For example, the carbonate ion (CO 32-) exhibits resonance, resulting in delocalized electrons and a reduced dipole moment compared to a single Lewis structure.
Intermolecular Forces
Intermolecular forces, such as dipole-dipole interactions and hydrogen bonding, can influence the polarity of a molecule.
- Dipole-dipole interactions:Attractive forces between polar molecules with oppositely oriented dipole moments.
- Hydrogen bonding:A particularly strong dipole-dipole interaction that occurs between molecules containing hydrogen bonded to highly electronegative atoms.
These intermolecular forces can enhance the polarity of a substance by aligning polar molecules or forming hydrogen bonds between them.
FAQ Compilation
What is the significance of molecular geometry in determining polarity?
Molecular geometry plays a crucial role in determining polarity because it influences the distribution of electrons within the molecule. Symmetrical molecular geometries, such as tetrahedral or octahedral, tend to cancel out the individual bond polarities, resulting in a nonpolar molecule.
In contrast, asymmetrical molecular geometries, such as bent or linear, can lead to a net dipole moment due to the uneven distribution of electrons.
How does electronegativity affect molecular polarity?
Electronegativity measures the ability of an atom to attract electrons towards itself. When atoms with different electronegativities are bonded, the more electronegative atom will pull the shared electrons closer to itself, creating a polar bond. The polarity of the individual bonds within a molecule contributes to the overall molecular polarity.
What is resonance, and how does it influence molecular polarity?
Resonance occurs when a molecule can be represented by multiple Lewis structures that differ in the placement of electrons. Resonance structures contribute to the overall polarity of the molecule by delocalizing the electrons, reducing the separation of charges and, consequently, the dipole moment.