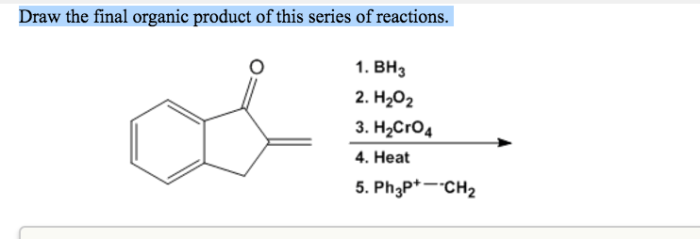
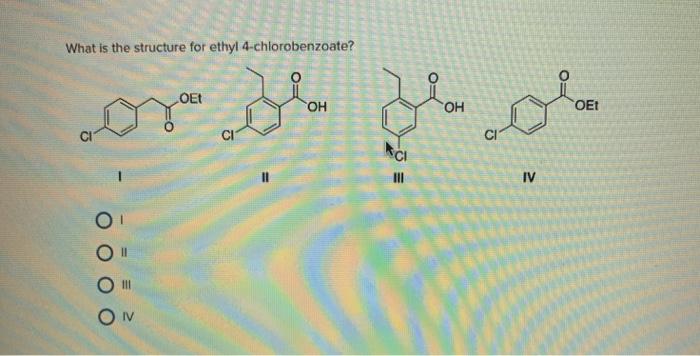
What is the structure for ethyl 4-chlorobenzoate – Ethyl 4-chlorobenzoate, an intriguing chemical compound, invites us on a journey to unravel its intricate structure. This guide delves into the molecular intricacies of this substance, exploring its physical and chemical properties, synthesis methods, diverse applications, and essential safety considerations.
Our exploration begins with a detailed examination of ethyl 4-chlorobenzoate’s molecular makeup, unraveling the arrangement of atoms and bonds that define its unique identity. We will then embark on a comprehensive analysis of its physical and chemical characteristics, gaining insights into its appearance, odor, reactivity, solubility, and stability.
Chemical Structure
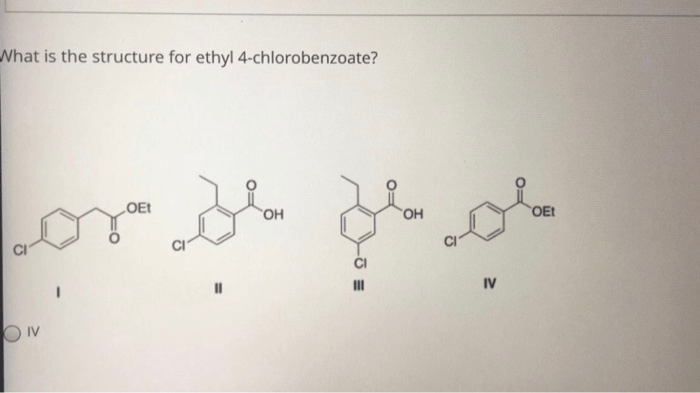
Ethyl 4-chlorobenzoate has a molecular formula of C9H9ClO2. It consists of a benzene ring with a chlorine atom attached to the fourth carbon atom. An ethyl group (-CH2CH3) is attached to the carbonyl group (-COOH) on the first carbon atom.
Physical and Chemical Properties
Ethyl 4-chlorobenzoate is a colorless liquid with a faint aromatic odor. It has a melting point of19.5 °C and a boiling point of 249.2 °C. It is slightly soluble in water and soluble in organic solvents such as ethanol and ether.
Ethyl 4-chlorobenzoate is a reactive compound that can undergo hydrolysis, reduction, and other reactions.
Synthesis and Production
Ethyl 4-chlorobenzoate can be synthesized by the reaction of 4-chlorobenzoyl chloride with ethanol in the presence of a base such as pyridine. It can also be produced by the Friedel-Crafts acylation of benzene with 4-chlorobenzoyl chloride. Industrially, ethyl 4-chlorobenzoate is produced by the reaction of 4-chlorobenzoic acid with ethanol in the presence of an acid catalyst.
Applications and Uses: What Is The Structure For Ethyl 4-chlorobenzoate
Ethyl 4-chlorobenzoate is used as an intermediate in the synthesis of dyes, pharmaceuticals, and other chemicals. It is also used as a plasticizer, a solvent, and a flavoring agent.
- In the pharmaceutical industry, ethyl 4-chlorobenzoate is used as an intermediate in the synthesis of ibuprofen and other non-steroidal anti-inflammatory drugs (NSAIDs).
- In the dye industry, ethyl 4-chlorobenzoate is used as an intermediate in the synthesis of azo dyes.
- In the plastics industry, ethyl 4-chlorobenzoate is used as a plasticizer for PVC and other plastics.
- In the flavor and fragrance industry, ethyl 4-chlorobenzoate is used as a flavoring agent in perfumes, cosmetics, and food products.
Safety and Handling
Ethyl 4-chlorobenzoate is a toxic and corrosive compound. It can cause skin irritation, eye damage, and respiratory problems. It is important to wear protective clothing and to work in a well-ventilated area when handling ethyl 4-chlorobenzoate. In case of contact with the skin or eyes, rinse immediately with plenty of water and seek medical attention.
Ethyl 4-chlorobenzoate should be stored in a cool, dry place away from incompatible materials such as strong acids and bases.
Query Resolution
What is the molecular formula of ethyl 4-chlorobenzoate?
C9H9ClO2
What is the appearance of ethyl 4-chlorobenzoate?
Colorless to pale yellow liquid
What is the melting point of ethyl 4-chlorobenzoate?
-23.5 °C
What are the applications of ethyl 4-chlorobenzoate?
Pharmaceuticals, fragrances, and dyes